Reaction Mechanisms
Gain Insight into Molecular-Level Steps
We have a long and fruitful collaboration with chemists and chemical engineers at the KU Center for Environmentally Beneficial Catalysis (CEBC) who develop catalysts and reaction systems for the production of industrially important chemicals. One of the ways in which we have contributed to these efforts is by mapping reaction pathways for the processes of interest. Our overarching goal in this work is to gain insight into the molecular-level steps of the reaction pathway to develop design principles - i.e., to predict how one could modify the rate or selectivity of a reaction by changing a reactant or property of the catalyst.
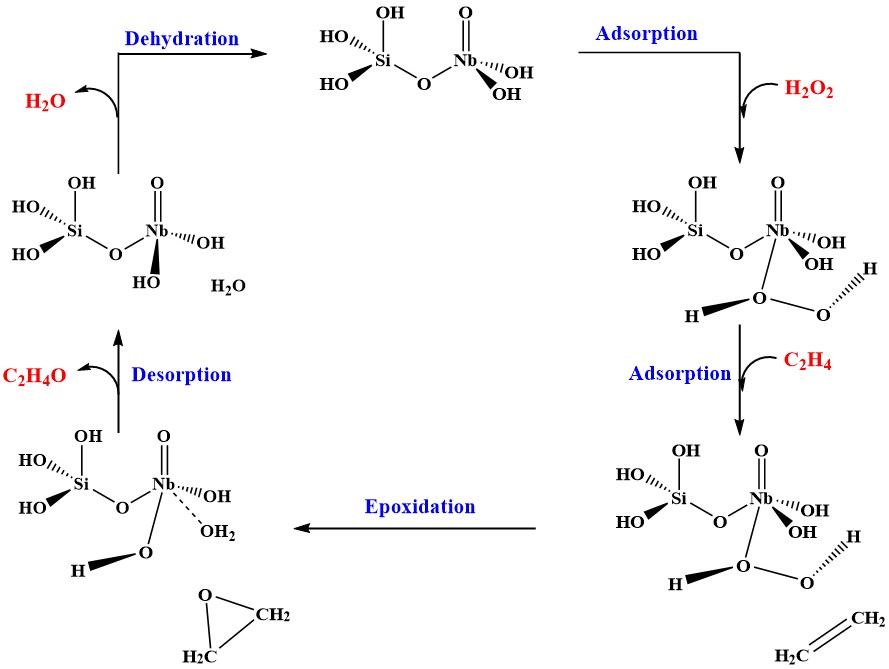
One of the recent systems we have worked on is the epoxidation of ethylene to ethylene oxide by Nb-doped mesoporous silica catalysts. This is one of the most significant industrial processes for producing commodity chemicals and a large contributor to greenhouse gas production. On the other hand, the Nb-doped mesoporous catalysts developed by the Subramaniam group at the CEBC generate no CO2 using hydrogen peroxide (H2O2) as the oxidant. However, they exhibit hydrogen peroxide decomposition and metal leaching, which need to be avoided. We mapped out the reaction pathway (shown below) using density functional theory (DFT) calculations and identified mechanisms for the undesired leaching and H2O2 decomposition that were associated with Bronsted acid sites, Nb-OH. Chemical modifications of the catalysts to neutralize these sites lead to significant reductions in the leaching and H2O2 decomposition.
DFT Calculations
We also used DFT calculations to investigate the (numerous) pathways in olefin ozonolysis to produce aldehydes and carboxylic acids. This can be used as an industrial process in the liquid phase, but the same chemistry is important in the (gas-phase) atmosphere where the functionalized products take part in the production of aerosol particles. Thus, it is of wide and varied interest. We showed that carboxylic acids can catalyze some key transformations in the rich ozonolysis chemistry. In one example, we predicted that a carboxylic acid could catalyze the isomerization of a Criegee intermediate to a vinyl hydroperoxide and the reaction was later demonstrated by the Lester group at the Univ. of Pennsylvania as shown.
Relevant References
Swarup K. Maiti, Anand Ramanathan, Ward H. Thompson, and Bala Subramaniam, Industrial & Engineering Chemistry Research56, 1999-2007 (2017). "Novel Strategies to Passivate Bronsted Acidity Enhances Hydrogen Peroxide Utilization and Reduced Metal Leaching in Ethylene Epoxidation"
Tapan Maji, Camina H. Mendis, Ward H. Thompson, and Jon A. Tunge, Journal of Molecular Catalysis A: Chemical424, 145-152 (2016). "Evidence for Isomerizing Hydroformylation of Butadiene. A Combined Experimental and Computational Study"
Pansy D. Patel, Brian B. Laird, and Ward H. Thompson, Journal of Molecular Catalysis A: Chemical424, 1-7 (2016). "A Density Functional Theory Study of Ethylene Epoxidation Catalyzed by Niobium-doped Silica"
Wenjuan Yan, Anand Ramanathan, Pansy D. Patel, Swarup K. Maiti, Brian B. Laird, Ward H. Thompson, and Bala Subramaniam, Journal of Catalysis336, 75-84 (2016). "Mechanistic Insights for Enhancing Activity and Stability of Nb-incorporated Silicates for Selective Ethylene Epoxidation"
Fang Liu, Yi Fang, Manoj Kumar, Ward H. Thompson, and Marsha I. Lester, Physical Chemistry Chemical Physics17, 20490-20494 (2015). "Direct Observation of Vinyl Hydroperoxide"
Manoj Kumar, Daryle H. Busch, Bala Subramaniam, and Ward H. Thompson, Physical Chemistry Chemical Physics16, 22968-22973 (2014). "Barrierless Tautomerization of Criegee Intermediates via Acid Catalysis"
Manoj Kumar, Daryle H. Busch, Bala Subramaniam, and Ward H. Thompson, Journal of Physical Chemistry A118, 5020-5028 (2014). "Organic Acids Tunably Catalyze Carbonic Acid Decomposition"
Manoj Kumar, Daryle H. Busch, Bala Subramaniam, and Ward H. Thompson, Journal of Physical Chemistry A117, 9701-9711 (2014). "Role of Tunable Acid Catalysis in Decomposition of α-Hydroxyalkyl Hydroperoxides and Mechanistic Implications for Tropospheric Chemistry"